Hydrogen Embrittlement and Stress Corrosion Cracking Protection
Why Electroplated Aluminum
Materials and mechanical engineers struggle with the protection of high-strength components against the double-edged sword of process and field hydrogen embrittlement. AlumiPlate’s world-class engineering has been developing solutions for tough aerospace problems for over 25 years. Electroplated aluminum is the clear solution to the protection struggle for flight-safety critical and controlled aerospace components.
Commercial and military aircraft include flight safety critical assemblies that are not redundant and undergo immense scrutiny during processing. Failure of such parts may lead to catastrophic loss of the aircraft. High-strength materials are required for critical load-bearing components. These materials are extremely sensitive to hydrogen embrittlement (HE) related to plating processes or from in-service corrosion, especially when under load.
The plating process is meant to enhance the material’s corrosion resistance but can instead reduce the material’s toughness if done incorrectly. In other words, the protective coating may deleteriously impact mechanical properties and performance. Post-plating “bakes” are prescribed to control HE related to plating processes. However, there is no prescription from HE that may occur in the field since hydrogen is a byproduct of corrosion.
Electroplated aluminum is unmatched amongst protective aerospace coatings for its ability to minimize HE concerns from both plating and in-field corrosion. Let our plating engineers design a customized solution for your application using our unique solvent-based aluminum electroplating technology.
Aluminum Plating For Hydrogen Embrittlement Control
DoD aerospace OEM testing has confirmed electroplated aluminum as the best choice for high-strength components used in demanding applications (1). Landing gear, pins, fasteners, actuators, pistons, cylinders, gear housings and other high-strength parts serve a critical structural or load-bearing purpose that is non-redundant in many cases. Failure of these components may lead to catastrophic failure of the entire vehicle and may endanger its occupants.
Critical and flight-safety parts are ideally suited for the aluminum electroplating process. Mechanical testing for multiple aerospace and ground vehicle programs found no HE, nor degradation of mechanical properties or a fatigue debit associated with water-based platings.
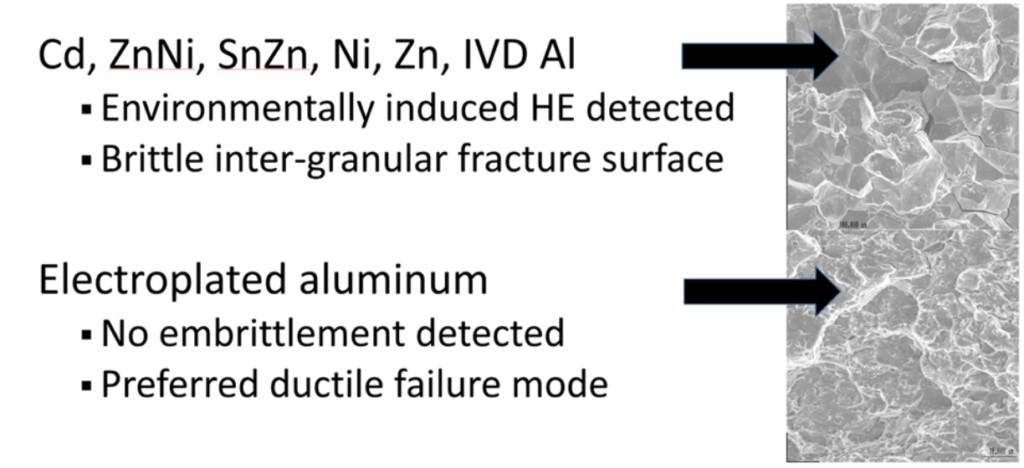
Figure 1: Metallographic analysis shows no embrittlement of aluminum-plated high-strength steel. In contrast, steel coated with cadmium, zinc, zinc-nickel, tin and organics coatings exhibit intergranular cracking characteristic of embrittlement.
Electroplated aluminum is the best coating option for high-strength steel, stainless steel, titanium, aluminum and structural metals sensitive to hydrogen embrittlement (HE). These materials require coatings to protect them from the service environment. However, the coating process and the coatings themselves may lead to hydrogen embrittlement of the substrate.
Hydrogen embrittlement reduces the toughness of high-strength materials making them prone to premature failure at lower than design stresses. A more perturbing technical factor is that failure associated with hydrogen embrittlement is often sudden and catastrophic, with fast crack propagation and fracture at stress levels much lower than the material’s intrinsic fracture strength.
A mechanism for HE involves the absorption of mono-atomic hydrogen (protons or H+cations). High enough concentrations of H+ can result in a combination of two hydrogen ions into a gaseous H2 molecule. Hydrogen molecules can pool together and create pressure stresses within the material. These stresses lower the material’s toughness, its ability to limit crack growth, making them more brittle. High-strength steel springs have been known to audibly pop from cracking due to hydrogen embrittlement.
Aluminum Plating Minimizes Hydrogen Embrittlement Exposure
Plating processes play a significant role in embrittlement of high-strength components. Electroless and electrolytic aqueous plating processes like cadmium, zinc, zinc-nickel, nickel, tin-zinc and tin generate hydrogen during the pre-treatment and plating steps. Baths can include additives to lower hydrogen generation, but hydrogen is always present during wet plating processes. Acid activation steps and the actual plating bath generate hydrogen which can be absorbed into the bulk material.
High-strength components are typically heat-treated after water-based plating to disperse or drive out the hydrogen. A 23-hour “HE bake” at 375° F is commonly performed on critical components after cadmium or nickel plating. However, even after the “HE bake,” latent hydrogen may remain in the substrate and impact its mechanical properties.
The aluminum electroplating process mitigates HE concerns. High-strength steels are coated using a simple two-step process consisting of a blast followed by aluminum plating, with minimum cleaning and degreasing pre-treatments.
The non-aqueous aluminum plating process is aprotic; it does not contain any free hydrogen (protons or H+). It is simply not possible to embrittle high-strength materials during aluminum electrodeposition.
Aluminum Plating Limits Environmentally Assisted Cracking and Stress Corrosion Cracking
High-strength components under service stresses in corroding environments can be protected from field embrittlement with pure aluminum electroplating. Cadmium, zinc-nickel and IVD aluminum exhibited hydrogen embrittlement failures from corrosion while under load.
High-strength steels may suffer from HE during service (referred to as “field embrittlement” or “field re-embrittlement”).
Mechanical testing indicates electroplated aluminum is the only coating that reduces the onset of environmentally assisted cracking and stress corrosion cracking “in the field.” Aluminum-plated steel subjected to electromechanical testing failed in a desirable and controlled ductile mode, with no evidence of embrittlement. High-strength steel plated with cadmium, zinc-nickel, nickel, zinc, tin, tin-zinc and aluminum-filled epoxies failed at lower-than-expected loads in a brittle manner, associated with field embrittlement.
In service, coated high-strength materials are still at risk of hydrogen embrittlement. The usual purpose of a coating is to retard, limit and slow corrosion of the base metal. Barrier coatings prevent corrosion until they are breached, and the corrosive environment interacts with the substrate. Sacrificial coatings offer additional electrochemical (galvanic) protection by preferably dissolving and slowing material corrosion, even after they are breached. In both cases, corrosion ultimately takes place leading to hydrogen evolution. Formation of free hydrogen is always associated with the reduction and oxidation reactions from environmental corrosion, from water or moisture (H2O) or other corroding environments such as SO2 or CO2.
Free hydrogen adsorption at the surface may lead to hydrogen embrittlement from exposure to the environment. When this type of embrittlement leads to component failure, it is often called environmentally assisted cracking (EAC).
High-strength components under load are even more at risk from hydrogen embrittlement due to in-service corrosion. High stress and corrosion are conjoint processes that can lead to fracture; cracks may initiate spontaneously and grow and propagate under the influence of corrosion (2). Stress corrosion cracking (SCC) of in-service components is characterized by premature failures at low loads, or failures only seen along with corrosion.
Electroplated Aluminum Protects High-Strength Steels Susceptible to Field Embrittlement
In service, aluminum-plated high-strength components showed low propensity to field embrittlement, stress corrosion cracking and environmentally assisted cracking (EAC). In side-by-side chemical-mechanical tensile testing against electroplated aluminum, cadmium, zinc-nickel and IVD aluminum-coated components exhibited hydrogen embrittlement in chemical-mechanical testing. Parts protected by electroplated aluminum exhibited no embrittlement under load in a corrosive cell.
Rising-Step-Load (RSL) testing of various coatings indicated electroplated aluminum is the best option for protecting steel susceptible to field embrittlement failures. RSL testing measured the coated specimen fracture strength while exposed to 3.5% NaCl solution. The mechanical and electrochemical response of the coating and substrate can be measured in this manner. The test is representative of the behavior of high-strength steel under load in a corrosive environment where salt water is present.
The propensity of the coated steel to hydrogen embrittlement was evaluated based on the fracture strength and the fracture surface. Fracture strength was reported as a threshold ratio: fracture stress of coated specimen / fracture stress of uncoated specimen. Specimens electroplated with aluminum failed at stresses close to those of unplated specimens. Specimens plated with other coatings failed at lower thresholds. Results are summarized in Figure 2.
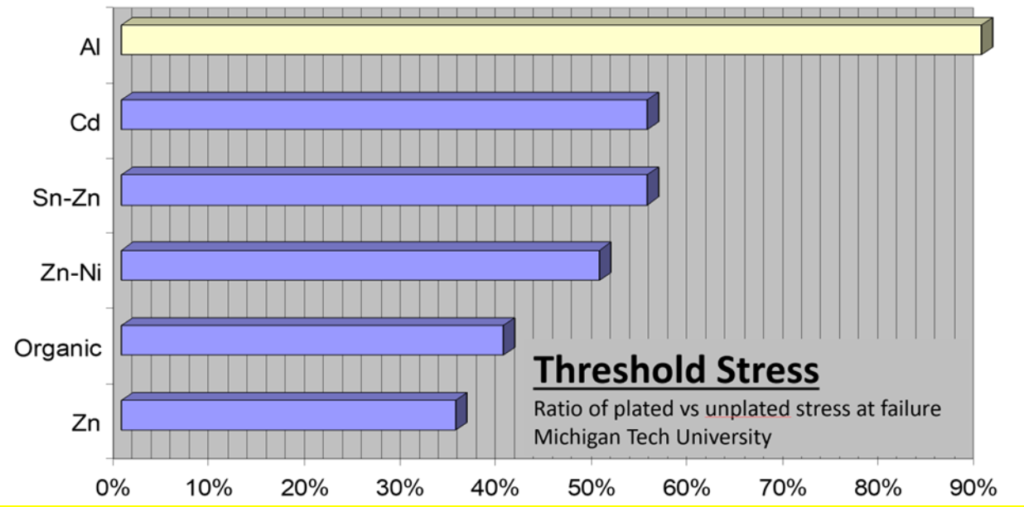
Figure 2: Electromechanical testing in 3.5% NaCl solution of high-strength steel with various coatings. Electroplated aluminum stands out with similar failure same stress as the baseline uncoated specimen.
More significantly, fracture surfaces for electroplated aluminum specimens showed the expected dimpled “taffy apple,” ductile fracture surface. Specimens with other coatings exhibited brittle fracture with a “rock candy” surface indicative of intergranular cracking from hydrogen embrittlement. See Figure 1 for representative micrographs.
This study conclusively identifies electroplated aluminum as the premier protective coating for critical high-strength steel components.
Sources:
(1) NAVAIR Mechanical Testing, High Strength Steel Joint Test Protocol (HSS JTP), F-35 Lightning II Program, Cd Alternative Coating Corrosion Performance on 4340 Steel, NACE, December 2007, P1792.
(2) West, John M., Basic Corrosion and Oxidation, Second Edition, Ellis Horwood Limited
(3) Zhang, Haidong, Susceptibility of Coated Steel to Environment-Induced Failure, Michigan Technological University
We Are Here To Help With Your Needs!
Contact us for more information about hydrogen embrittlement solutions or to discuss your application in more detail.